Research and Woodruff Health Sciences IT


In his sixth year in the role of Deputy Chief Information Officer, Marc Overcash leads the Research and Woodruff Health Sciences IT Division in the advancement of research through the application of information technology. Marc also serves as the Assistant Dean of IT in the School of Medicine, Adjunct Faculty in Public Health Informatics in the Rollins School of Public Health, and co-director of the Biomedical Informatics Program for the Atlanta Clinical and Translational Science Institute.
The Research & Woodruff Health Sciences IT (R-WIT) division continued to advance the core research and health science platforms in support of faculty in their research and education. In both the research and the health science domains, FY12 heralded several exciting initiatives designed with and for faculty, staff, and students. Here are some of the highlights:
Web Design
The Flour Fortification Initiative (FFI) is a network of global partners encouraging countries to add essential vitamins and minerals to flour. Among the partners are flour millers, scientists, government ministries, and non-governmental organizations. Their governing body includes members from the Centers for Disease Control and Prevention (CDC), Unicef, the World Health Organization (WHO), Emory School of Public Health, Cargill, the Global Alliance for Improved Nutrition (GAIN), Fleishman-Hillard, etc. (http://ffinetwork.org/about/management/index.html).
The customer asked R-WIT to build a website to promote and enhance Flour Fortification worldwide, targeted at Ministers of Health in developing nations, global partners, flour millers in countries that have no mandatory fortification, large-scale donors, and the public at large with interest in the subject matter. The goals of the customer were to engage and educate site visitors about Flour Fortification, and encourage them to adopt mandatory fortification standards. The project leverages FFI-managed data input into RedCap, which is then exported on a nightly basis to both the mapping vendor for data display in their interfaces, as well as an Oracle database for implementation in the web interface.
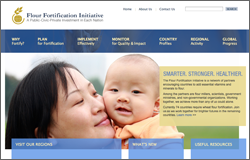 The project also includes an LDAP-secured intranet portal used for push-communications to global partners in the field, as well as a small-scale document repository for subject matter-relevant research, FFI events information, and data sheets on a per-country basis.
The project also includes an LDAP-secured intranet portal used for push-communications to global partners in the field, as well as a small-scale document repository for subject matter-relevant research, FFI events information, and data sheets on a per-country basis.
This project is a great example of cross-functional collaboration within the division, and partnerships with UTS and external vendors. The team addressed several issues in integrating multiple systems in a sustainable long-term solution. The project provides valuable return on investment for the customer, and their website traffic to-date achieves in a month what previously took them six months.
Application Development
The Food and Drug Administration (FDA) recently approved the drug Belatacept (nulojix) for the prevention of graft rejection after a kidney transplant – the first new class of drugs since the 1990's for transplants. Drs. Chris Larsen and Thomas Pearson of Emory University played key roles in the development of this immune tolerance drug.
 As research continues on the drug, the Transplant Center reached out to R-WIT to design a more efficient way to track and manage the adherence of patients to the belatacept protocol. Working closely with the Transplant Center's Cynthia Devroy, the application, affectionately named Badger, was designed and deployed within a four-month timespan. The application enables administrators to generate a schedule for each patient based on the version of the belatacept protocol. In addition, the application can quickly enable administrators to see any deviations from the protocol so they can be addressed.
As research continues on the drug, the Transplant Center reached out to R-WIT to design a more efficient way to track and manage the adherence of patients to the belatacept protocol. Working closely with the Transplant Center's Cynthia Devroy, the application, affectionately named Badger, was designed and deployed within a four-month timespan. The application enables administrators to generate a schedule for each patient based on the version of the belatacept protocol. In addition, the application can quickly enable administrators to see any deviations from the protocol so they can be addressed.
Although designed for the belatacept protocol, Surgery is already thinking of other research and standard of care protocols that can be adapted for the application. Badger attempts to optimize the capture and management of data and present it to clinical investigators so they are able to concentrate more on quality of care and discovery.
Sponsored Award Management (SAM) Kiosk
Transparency, efficiency, and better customer support. These are the underlying drivers that Holly Sommers, Urvi Patel, and Evelyn Balabis wanted when they thought about how to improve key processes within the research administration area. Currently, these high priority processes were all done through paper forms and manual routing, so it was time-consuming to determine who was working on which request, how long they have had the request, and when they anticipate it completing. It felt like things could be done better.
In the Fall of 2012, the SAM Kiosk, will be released. The Kiosk creates an electronic system for research administration to establish electronic forms, enable investigators and research administrators to check on the status of the requests, and provide leadership with the data to help manage the overall process across the system. Instead of calling research administration each time they want to know where their request is, individuals can simply log into the Kiosk and find out - no calls needed. Leadership in research administration can have more data that can help them distribute the workload, identify rate limiting factors, and expand upon techniques that are working well. From a technology perspective, this application paves the way for Emory Commons, the investigator portal, and provides a reusable form engine that can be leveraged in multiple applications.
Advancing the Missions
In FY13, the Research and Woodruff Health Sciences IT Division will continue to expand our services to investigators, providing them with platforms and applications to facilitate the conduct of research.
Two high priorities will be building out the Emory Commons and the Clinical Trials IT environment. Partnering with investigators, research administrators, research deans, and the Office of Research Administration, we will deploy the first phase of the Emory Commons, a portal designed for the researcher. When a researcher logs in, s/he will be presented with key information about their proposals and awards, drawing information in near real time from authoritative systems. Investigators will be able to see across the ecosystem of systems what the status of their proposals is as well as have a single place to aggregate their research awards and proposals information. In the realm of clinical trials IT, we plan to work with the clinical trials community to document their needs and lay out a roadmap of systems and tools to help them conduct their clinical trials.
 As we establish the roadmap, we plan on tackling emerging issues and obstacles, such as assuring compliance with the Federal Information Security Management Act (FISMA) and Part 11 requirements. In FY12 we have seen a remarkable growth in the use of our core services, such as application development, database work, electronic case report forms, laboratory information management systems, and data management activities. In FY13, we anticipate that growth to continue and look forward to helping in the advancement of discovery here at Emory.
As we establish the roadmap, we plan on tackling emerging issues and obstacles, such as assuring compliance with the Federal Information Security Management Act (FISMA) and Part 11 requirements. In FY12 we have seen a remarkable growth in the use of our core services, such as application development, database work, electronic case report forms, laboratory information management systems, and data management activities. In FY13, we anticipate that growth to continue and look forward to helping in the advancement of discovery here at Emory.
Marc Overcash
Deputy Chief Information Officer, Research and Woodruff Health Sciences IT
"The FFI site is an example of the ability of the team to provide creative, innovative solutions that incorporate and extend technology and branding outside of the Emory template."
Rachelle Willoughby
Senior Manager, Web Development, R-WIT